

34th Annual Scientific Meeting proceedings
Date/Time: 06-07-2024 (09:00 - 09:30) | Location: Auditorium 2
Most of the joint-related problems accounting for an important economic loss in the equine industry are related to trauma, congenital conditions such as osteochondrosis and degenerative disorders leading to various grade of osteoarthritis (1). Destroyed hyaline cartilage never naturally repairs itself identically and the resulting repair tissue from non-surgical or surgical treatment options cannot be compared with a structure and a function approximating a native cartilage (2).
Because the majority of joint disorders are, or induce, an inflammatory condition, non-surgical options are focused on intraarticular medications such as anti-inflammatory drugs including PRP, IRAP and hyaluronic acid (3). More recently intraarticular mesenchymal stem cells (MSCs) administration was described (4). These cells act via their secretome to counter inflammation (5). However, none of these intraarticular medications helps to resurface a degenerated hyaline cartilage.
To achieve joint resurfacing with hyaline cartilage, it is necessary to surgically approach the cartilage lesion to be repaired with the cellular implant that have to adhere to the defect. Surgical options to help repair a destroyed cartilage are grouped into three categories palliative, reparative and restorative (2,6). The two first categories include debridement of fibrous tissue and loose bone in any full-thickness cartilage defect, chondroplasty to remove partial-thickness fibrillation, subchondral bone drilling, microfracture and micro-picking (1). Unfortunately, the resulting repair tissue after such treatments cannot be compared with a native cartilage (2). Restorative techniques are tissue-based transplantation procedures (6) such as cartilage flaps reattachment with polydioxanone pins (7), various autologous osteochondral grafting (8, 9,10) and chondrocyte implantation in a matrix carrier vehicle (10, 11, 12).
Recent technological developments have enabled to assess in horses fully dedifferentiated adult chondrocytes which are redifferentiated to engineer hyaline cartilage microtissues called Cartibeads (13). Cartibeads are composed of active chondrocytes embedded in their own released hyaline matrix characterized by the presence of collagen type II and glycosaminoglycan. This technology allows large cartilage lesions to be treated. In an ongoing phase I/IIa clinical trial with ten human patients cartilage lesions ranging from 1.5 to 11 cm2 are being treated with autologous Cartibeads (14) and cartilage regeneration based on MRI monitoring is observed (16). In minipigs preclinical data using autologous Cartibeads (15) are encouraging and show cartilage regeneration with hyaline quality maintained after six months. Preliminary results obtained in horses following the use of allogeneic Cartibeads is used to describe this resurfacing technique and discuss its limitations in this species.
Production of cartibeads
Equine allogeneic Cartibeads were made from the chondrocytes of an 8-year-old donor horse and transplanted in other adult horses. The chondrocytes were extracted from the donor horse cartilage by enzymatic action and cultured for expansion in a first step with growth factors (Figure 1). These cells dedifferentiate meaning they lose their chondrogenic characteristics very rapidly. During a second step they were cultured in another culture medium, allowing them to redifferentiate and produce the hyaline matrix. During the third step, the Cartibeads are formed by the aggregation of cells collected during the second step of culture. Cells after step 1 are stored frozen in a Master Cell Bank (MCB) and used to produce a Working Cell Bank (WCB) in the step 2. Cartibeads in step 3 are directly produced from the WCB and can be shipped at room temperature and stored at 4°C for up to 3 weeks.
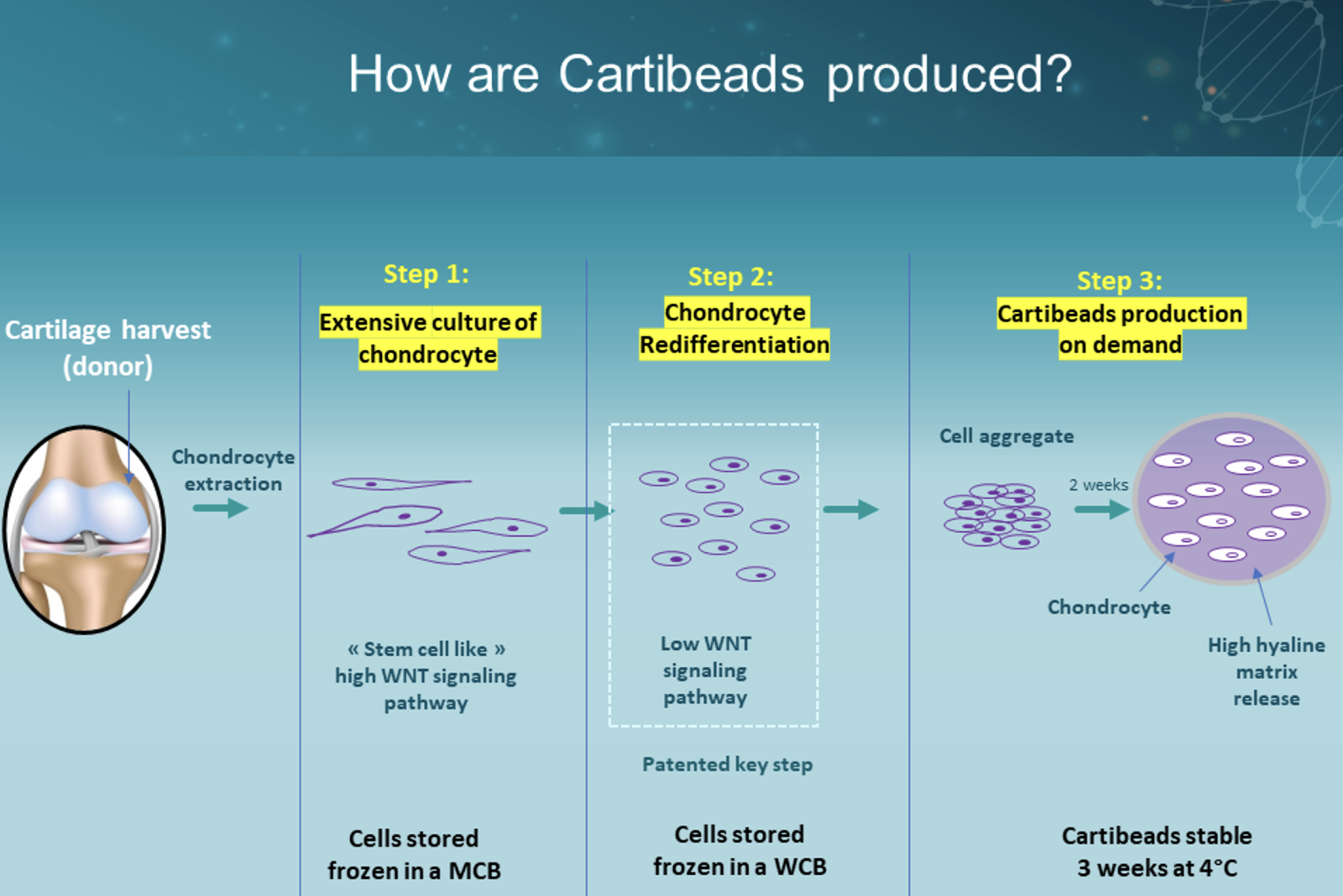
Figure 1 - Cartibeads production steps. MCB = Master Cell Bank; WCB = Working Cell Bank
CARTILAGE RESURFACING using Cartibeads in horses
With the horse under general anaesthesia, an arthrotomy of the affected joint was performed to obtain the best approach to the area involved by a cartilage defect. In the case of osteochondrosis dissecans, the fragments were removed before preparation of the cartilage defect which consist in removal of the calcified cartilage layer while maintaining the integrity of the subchondral bone (17). Stable cartilaginous edges perpendicular to the joint surface (18) were obtained using a specific set of chondrectom (Chondrectom™ Biovico, Poland). At this stage of the procedure, and before placing the desired number of Cartibeads, the cartilage defect was free of any liquid such as blood, lavage solution or synovial fluid. Cartibeads were transferred from their shipping-container into a stainless-steel bowl. The culture medium was removed and the Cartibeads rinsed with physiologic serum. The Cartibeads were recovered and placed dry on the lesion to be treated with a large curette if it was possible to bring the defect close to the arthrotomy skin incision. If it was too deep, the Cartibeads were placed in a bone pulp guide with its loader (Chondrectom™, Biovico, Poland) (Figure 2). Once the liquid flows out of the device, an obturator was advanced to place the Cartibeads into the cannula. The loader was removed and the pulp guide cannula positioned in regards of the defect, the Cartibeads were then pushed into it using the obturator. Once the lesion was completely covered with one or two layers of Cartibeads a thin layer of fibrin glue (Tisseel®, Baxter) was applied to hold them in place. For the next five minutes, the surgical site was kept as dry as possible to allow polymerization of the fibrin sealant which becomes less translucent (Figure 2). Excess fibrin was then gently removed from around the defect by cutting with an instrument such as a front chondrectom or an n°11 scalpel blade. The limb was gently extended and flex to ensure that the cartibeads remain in place before performing a standard arthrotomy closure.
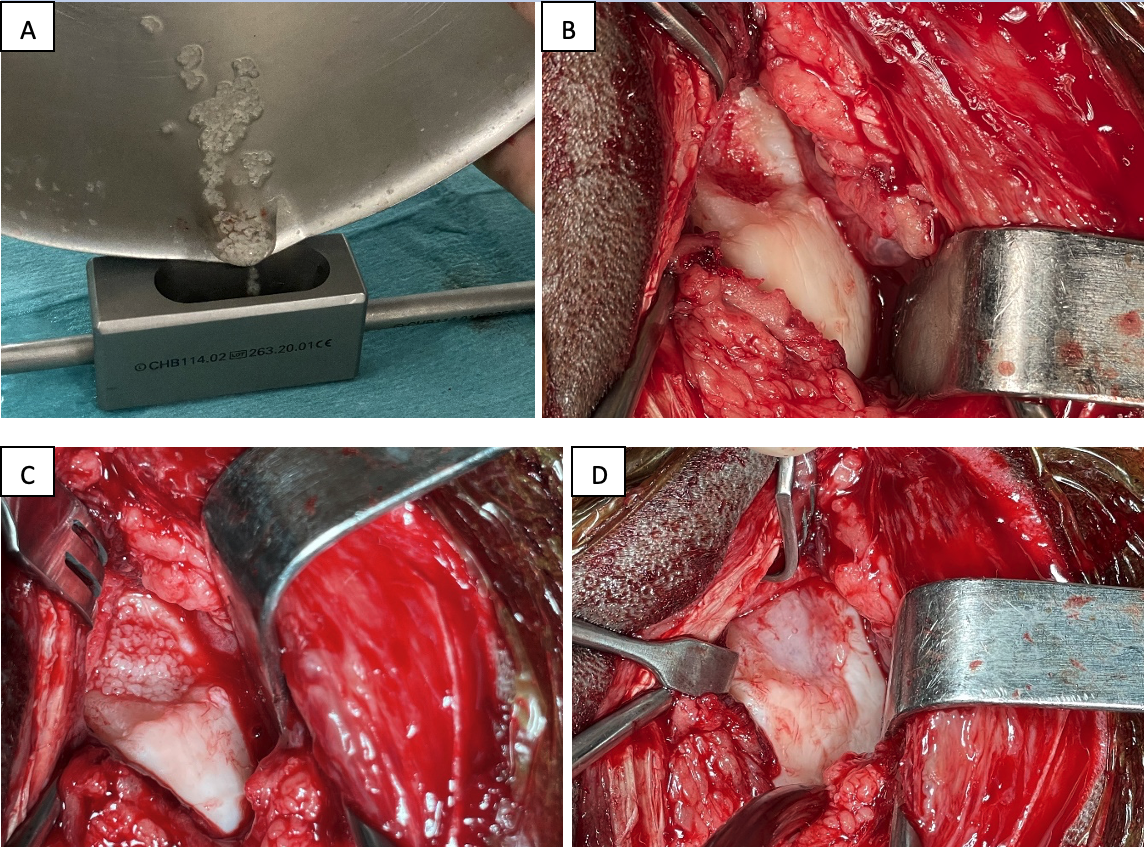
Figure 2 – Femoro-patellar lateral approach arthrotomy for cartilage resurfacing using Cartibeads in a 4-year-old Selle Français mare: (A) Cartibeads dumping into a bone pulp loader; (B) lateral ridge of the femur after debridement of an OCD lesion; (C) transplantation of the Cartibeads; (D) aspect of the resurfacing region after polymerization of the fibrin glue.
Recovery, post-operative care and follow-up
Depending on the joint involved in the resurfacing of its cartilage, it is necessary to assess, when possible, the feasibility of immobilizing it for at least the period of the horse's recovery from anaesthesia. The aim of this action is to prevent the risk of delamination due to implant friction. For example, when resurfacing a fetlock (Figure 3), a half-limb cast or Kimzey splint was applied for the recovery period, and removed within a week after the resurfacing procedure. In the event of resurfacing a defect in the stifle (Figure 2) no immobilization of the joint was possible. The rehabilitation protocol consists of complete rest in the stall for four weeks before being walked twice a day for 15 minutes for a period of eight weeks. At the end of this 12 weeks convalescence period, the horse was gradually returned to their intended activity. This prescription of a long convalescence time was linked to the healing of the arthrotomy site, in the absence of data on the rest time required for proper implant integration.
Follow-up arthroscopy of a 3-year-old Selle Français gelding that had undergone joint resurfacing of an osteochondritis dissecans lesion of the midsagittal ridge of the third metacarpal bone in both front fetlocks (Figure 3) showed complete coverage of the subchondral bone by cartilage-like tissue. The horse then entered its breaking-in period before beginning mounted training for show jumping. At nine months post-surgery, no lameness or front fetlock abnormalities were observed.
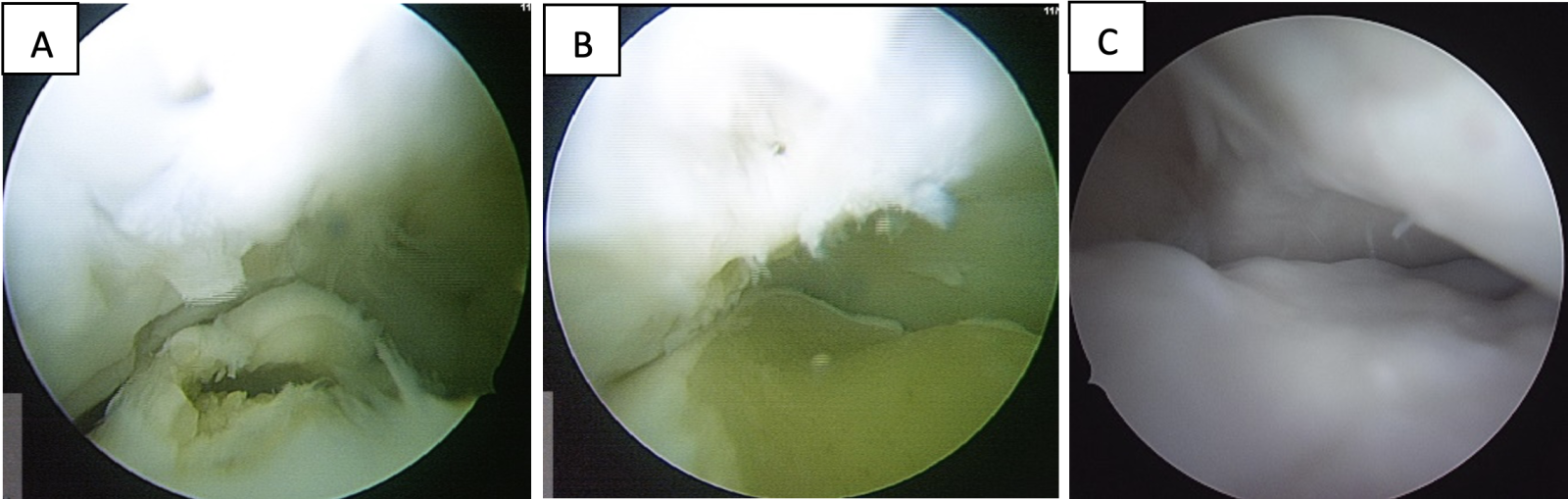
Figure 3 – Metacarpophalangeal joint arthroscopical findings in a 3-year-old Selle Français gelding. (A) osteochondritis dissecans of the midsagittal ridge of the third metacarpal bone; (B) after debridement; (C) three months after Cartibeads transplantation.
Current limits of cartilage resurfacing using cartibeads in horses
It is important to carefully prepare the cartilage defect to be treated. When full exploration of a joint is required or multiple OCD fragments needs to be removed it is preferable to first approach the joint arthroscopically before deciding on the ideal arthrotomy approach for implanting Cartibeads the same day or in the next couple of weeks.
While it has been demonstrated that the cartilage obtained after autologous or allogeneic transplantation is hyaline (13, 16), the quantity and quality of hyaline cartilage produced in a clinical trial of horses after an allogeneic graft is unknown as no follow-up MRI assessment or biopsies have been performed in these animals dedicated to sporting activity. But follow-up arthroscopies of individuals demonstrate the presence of the graft, after Cartibeads transplantation of a spontaneous and an induced cartilage defect, respectively after 3 and 18 months. In humans, preliminary results at the six-month follow-up of a prospective clinical trial on patients with condylar or trochlear cartilage lesions shows good filling with autologous cartibeads and integration of the graft with hyaline-like cartilage quality assess by T2 mapping (14).
Whatever the joint to be treated, the surgical approach to the lesion site remains a challenge for the equine surgeon. For example, the fetlock cartilage is thinner than the human cartilage and techniques for resurfacing are demanding (2). In these cases, care must be taken to ensure that the Cartibeads do not exceed the normal thickness of the cartilage surrounding the lesion, otherwise they will be delaminated during joint movement. However, in the case of deep lesions, it is possible to apply two or three coats of Cartibeads.
The necessity of immediate weight-bearing post-operatively is the first risk for Cartibeads delamination. This is why it is sometimes advisable to apply some type of immobilisation to the operated limb while the patient is recovering from anaesthesia and for the first post-operative week. We also observed partial skin dehiscence at the site of a stifle arthrotomy where no immobilisation was possible. This complication could perhaps be avoided by arthroscopy with gas distension but the complexity of transplantation and the difficulty to reach some anatomical location with the Cartibeads requires adaptations to a standard arthroscopy.
PERSPECTIVE
Cartibeads are the first hyaline grade cartilage microtissue engineered from adult dedifferentiated chondrocytes with a proven safety profile (12). A clinical trial on five human patients with large cartilage lesions of the knee demonstrates the safety of Cartibeads and complete integration of the graft (14). In horses, this technique should be assessed as a therapeutic alternative in case of a cartilage defect of high motion joints to preserve its normal function. Based on one horse donor, unlimited production of cartilage microtissue can be produced with a standardized methodology (13) and transplanted in other individuals without the need of a scaffold at the exception of using a human fibrin glue to keep the transplant in place to promote Cartibeads attachment to the underlying subchondral bone. However, it is clear that a great deal of work remains to be done in equine surgery, particularly with regard to the quality oy hyaline cartilage produced, the surgical approach to transplantation, the ideal technique for recovery from anaesthesia and the rehabilitation protocol.
References
- McIlwraith CW, Nixon AJ, Wright IM. (2015) Arthroscopic methods for cartilage repair, In: Diagnostic and surgical arthroscopy in the horse. Elsevier 4th edition, pp 426-442
- Cokelaere S, Malda J, van Weeren R. (2016) Cartilage defect repair in horses: current strategies and recent developments in regenerative medicine of the equine joint with emphasis on the surgical approach. TVJ 214: 61-71
- Han W, Lv, Y, Wang Y, Zhao Z, Shi C, Chen X, Wang L, Zhang M, Wei B, Zhao X, Wang X. (2022) The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carb Polymers, https://doi.org/10.1016/j.carbpol.2021.118699
- Schnabel LV, Fortier LA, McIlwraith CW, Nobert KM. (2013) Therapeutic use of stem cells in horses: Which type, how, and when? TVJ 197: 570-577
- Mancuso P, Raman S, Glynn A, Barry F, Murphy M. (2019) Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Front Bioeng Biotechnol 7: 9, doi:10.3389/fbioe.2019.00009
- Hunziker EB, Lippuner K, Keel MJB, Shintani N. (2015) An educational review of cartilage repair: precepts & practice – miths & misconceptions – progress & prospects. Osteoarthritis Cartilage 23: 334-350
- Nixon AJ, Fortier LA, Goodrich LR, Ducharme NG. (2004) Arthroscopic reattachment of selected OCD lesions using resorbable polydioanone pins. Equine vet J 36: 376-383
- Hurtig MB. (1988) Experimental use of small osteochondral grafts for resurfacing the equine third carpal bone. Equine vet J Suppl 6: 23-27
- Bodo G, Hangudy L, Modish, Hurtig M. (2004) Autologous osteochondral grafting (mosaic arthroplasty) for treatment of subchondral cystic lesions in the equine stifle and fetlock joints. Vet Surg 33: 588-596
- Frisbie DD, Lu Y, Kawcak CE, Di Carlo EF, Binette F, McIlwraith CW. (2009) In vivo evaluation of autologous cartilage fragment-loaded scaffold implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sport Med 37: 71-80
- Hendrickson DA, Nixon AJ, Grande DA et al. (1994) Chondrocytes-fibrin matrix transplants for resurfacing extensive articular cartilage defects. J Orthop Res 12: 485-497
- Fortier LA, Lust G, Mohammed HO, Nixon AJ. (2002) Insulin-like growth factor-I enhances cell-based articular cartilage repair. J Bone Joint Surg 84: 276-288
- Kutaish H, Bengtsson L, Matthias Tscholl P, Marteyn A, Braunersreuther V, Guérin A, Béna F, Gimelli S, Longet D, Ilmjärv S, Dietrich PY, Gerstel E, Jaquet V, Hannouche D, Menetrey J, Assal M, Krause KH, Cosset E, Tieng V. (2022) Hyaline Cartilage Microtissues Engineered from Adult Dedifferentiated Chondrocytes: Safety and Role of WNT Signaling. Stem Cells Transl Med 11:1219-1231. doi: 10.1093/stcltm/szac074
- Kutaish H. (2024) Engineered hyaline Cartilage mini-grafts, a solution for the treatment of large cartilage lesions of the knee: A first-in-man study. Proceedings European Society for Sports Traumatology, Knee Surgery and Arthroscopy, Milano (Italy)
- Kutaish H, Tscholl PM, Cosset E, Bengtsson L, Braunersreuther V, Mor FM, Laedermann J, Furfaro I, Stafylakis D, Hannouche D, Gerstel E, Krause KH, Assal M, Menetrey J, Tieng V. (2023) Articular Cartilage Repair After Implantation of Hyaline Cartilage Beads Engineered from Adult Dedifferentiated Chondrocytes: Cartibeads Preclinical Efficacy Study in a Large Animal Model. Am J Sports Med 51: 237-249. doi: 10.1177/03635465221138099
- Tieng V., Bouddabous S, Kutaish H, Bengtsson de Barrio L, Lazeyras F, Braunersreuther V, Turchetto L, Dib Y, Moraca G, Sangiorgio A, Deabate L, Delcogliano M, Lepage O, Hannouche D, Gerstel E, Fucentese SF, Menetrey J, Tscholl PM. (2024) From autologous to allogeneic Cartibeads: A game-changing solution for addressing knee cartilage damage? Proceedings 7th International Cartilage Repair Society, Catania (Sicilia).
- Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. (2006) Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med 34: 1824-1831
- Rudd RG, Visco DM, Kincaid SA, Cantwell HD. (1987) The effects of bevelling the margins of articular cartilage defects in immature dogs. Vet Surg 16: 378-383